Key Takeaways:
- Iron overload happens when the body absorbs excessive iron, which can damage organs.
- Common symptoms include fatigue, joint pain, and skin changes.
- Early detection is key to preventing complications.
- Regular blood tests and dietary changes can help manage iron levels.
- Genetic factors like hereditary hemochromatosis increase the risk of developing iron overload.
What is Iron Overload?

Iron is essential for many body functions, but too much can be harmful. Iron overload, also known as hemochromatosis, occurs when the body absorbs more iron than it needs.
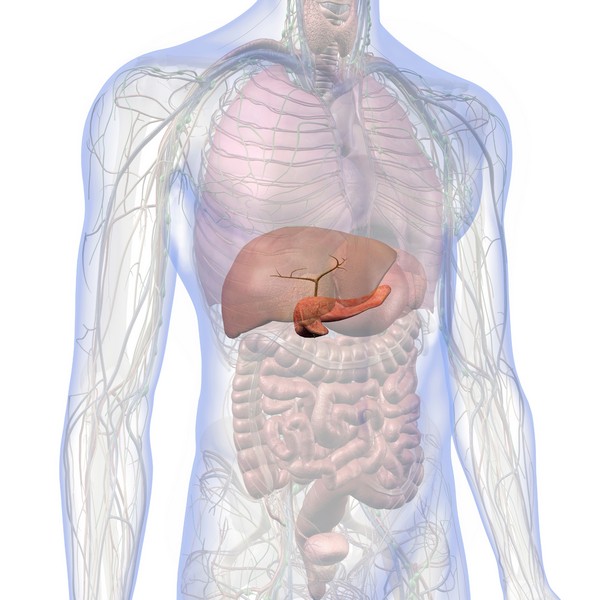
Over time, excess iron builds up in organs like the liver, heart, and pancreas, potentially causing serious health problems.
Iron overload is a condition where the body stores too much iron, leading to damage in vital organs. While some people absorb just the right amount of iron from their diet, those with iron overload absorb more than necessary.
Without treatment, this excess iron can cause conditions like liver disease, diabetes, heart problems, and arthritis.
Causes of Iron Overload
Hereditary Hemochromatosis
Hereditary hemochromatosis is a genetic disorder that causes the body to absorb more iron than it needs. It’s the most common cause of iron overload, particularly in people of Northern European descent.
Those with this genetic mutation are at a higher risk and may begin showing symptoms later in life.
Frequent Blood Transfusions
People who receive regular blood transfusions for conditions like anemia or thalassemia can develop iron overload. This happens because each transfusion adds more iron to the body, which the body cannot eliminate naturally.
Iron Supplements and Fortified Food
Iron supplements can quickly lead to iron overload, especially if someone is already at risk due to genetic factors. It’s important not to take iron-containing supplements.
Symptoms of Iron Overload
Chronic Fatigue and Weakness
One of the most common symptoms of iron overload is ongoing fatigue that doesn’t improve with rest. This happens because excess iron can damage organs, leading to a lack of energy.
Joint Pain
Iron can deposit in the joints, causing pain and stiffness. This joint pain may be mistaken for arthritis and is especially common in the hands and fingers.
Abdominal Pain and Liver Enlargement
Iron tends to accumulate in the liver, leading to discomfort or pain in the upper right side of the abdomen. Over time, the liver can become enlarged or damaged, increasing the risk of cirrhosis or liver cancer.
Skin Discoloration (Bronze or Gray Skin)
A unique symptom of iron overload is the development of bronze or gray skin. This discoloration is caused by iron deposits in the skin and is often a sign of advanced iron overload.
Heart Problems (Irregular Heartbeat)
Excess iron can damage the heart, leading to conditions like irregular heartbeats, heart failure, or cardiomyopathy. Managing iron levels is crucial to prevent these serious complications.
Diagnosis of Iron Overload
Blood Tests (Serum Ferritin, Transferrin Saturation)
Blood tests are the primary way to diagnose iron overload.
- Hemoglobin Measures the protein that carries oxygen and holds most of the iron in the body.
- Serum Iron Measures how well the body uses iron.
- Serum Ferritin Shows how much iron storage protein is in the body.
- Serum Transferrin Tracks the main protein that moves iron from tissues into the bloodstream for reuse.
- Total Iron-binding Capacity Indicates how much iron can be carried in the blood, showing available spaces for iron to bind.
Genetic Testing for Hemochromatosis
If hereditary hemochromatosis is suspected, genetic testing can confirm whether a person has the gene mutations responsible for the condition. This test is particularly important for those with a family history of iron overload.
Liver Function Tests and Imaging
Liver function tests help assess whether the liver has been affected by iron overload. In some cases, imaging tests such as an MRI or liver biopsy may be used to check for liver damage or scarring.
Complications of Untreated Iron Overload
Liver Disease (Cirrhosis, Liver Cancer)
Untreated iron overload can lead to serious liver damage, including cirrhosis and liver cancer. Early diagnosis and management can prevent these outcomes.
Diabetes
Iron buildup in the pancreas can interfere with insulin production, leading to diabetes. This is a common complication in people with advanced iron overload.
Heart Disease
Excess iron can damage the heart muscle, leading to heart failure, arrhythmias, or other heart-related conditions.
Joint and Bone Damage
Over time, iron deposits in the joints can lead to arthritis and bone damage. Proper treatment and management of iron levels can reduce the risk of long-term joint issues.
Prevention and Management of Iron Overload

Regular Blood Donation (Therapeutic Phlebotomy)
For people with hereditary hemochromatosis or iron overload, regular blood donation, known as therapeutic phlebotomy, is an effective way to reduce iron levels. By removing blood, the body uses excess iron to produce more red blood cells.
Dietary Adjustments
Managing iron intake is important for preventing further buildup. Avoiding fortified foods can help keep iron levels in check. It’s also recommended to never take any supplements containing iron.
Having good retinol and bioavailable copper levels is important for regulating iron metabolism. Bioavailable nutrient-dense food would provide all the requirements to properly balance iron levels.
Monitoring Iron Levels with Routine Testing
Routine blood tests are necessary for those at risk of or diagnosed with iron overload. These tests monitor iron levels and ensure they remain within a healthy range.
Conclusion
Iron overload is a serious condition, but with early detection and proper management, the risks of complications can be minimized. Understanding the symptoms and causes is key to preventing long-term organ damage. Regular blood tests, lifestyle adjustments, and medical treatments can help those at risk manage their iron levels effectively.
FAQs
What are the early signs of iron overload?
Early signs include chronic fatigue, joint pain, and skin discoloration. If you experience these symptoms, consult a healthcare provider.
Can iron overload be prevented?
For those with genetic risk factors, regular monitoring and managing iron intake can help prevent the buildup of excess iron. Therapeutic phlebotomy is also an effective prevention method.
How is hereditary hemochromatosis diagnosed?
Hereditary hemochromatosis is diagnosed through genetic testing, which looks for mutations in the HFE gene. Blood tests to measure iron levels are also used to confirm the condition.
Is it safe to take iron supplements without testing?
No, taking iron supplements without knowing your iron levels can lead to iron overload, especially if you are genetically predisposed. Always consult a doctor before taking iron supplements.
How often should iron levels be checked for those at risk?
Those at risk for iron overload should have their iron levels checked at least once a year. More frequent monitoring may be required if iron overload is diagnosed.
Research
Batey RG, Lai Chung Fong P, Shamir S, Sherlock S. A non-transferrin-bound serum iron in idiopathic hemochromatosis. Dig Dis Sci. 1980 May;25(5):340-6. doi: 10.1007/BF01308057. PMID: 7371472.
Bo, S., Durazzo, M., Gambino, R., Berutti, C., Milanesio, N., Caropreso, A., Gentile, L., Cassader, M., Cavallo-Perin, P. and Pagano, G., 2008. Associations of Dietary and Serum Copper with Inflammation, Oxidative Stress, and Metabolic Variables in Adults ,. The Journal of Nutrition, [online] 138(2), pp.305–310. https://doi.org/10.1093/jn/138.2.305.
Boddaert, N., Le Quan Sang, K. H., Rötig, A., Leroy-Willig, A., Gallet, S., Brunelle, F., Sidi, D., Thalabard, J., Munnich, A., & Cabantchik, Z. I. (2007). Selective iron chelation in Friedreich ataxia: Biologic and clinical implications. Blood, 110(1), 401-408. https://doi.org/10.1182/blood-2006-12-065433
Collins, J. F. (2021). Copper nutrition and biochemistry and human (patho)physiology. Advances in Food and Nutrition Research, 96, 311-364. https://doi.org/10.1016/bs.afnr.2021.01.005
DiNicolantonio, J.J., Mangan, D. and O’Keefe, J.H., 2018. The fructose–copper connection: Added sugars induce fatty liver and insulin resistance via copper deficiency. Journal of Metabolic Health, [online] 3(1).
https://doi.org/10.4102/jir.v3i1.43.
Fillebeen, C., Descamps, L., Dehouck, M.-P., Fenart, L., Benaïssa, M., Spik, G., Cecchelli, R. and Pierce, A., 1999. Receptor-mediated Transcytosis of Lactoferrin through the Blood-Brain Barrier. Journal of Biological Chemistry, [online] 274(11), pp.7011–7017. https://doi.org/10.1074/jbc.274.11.7011.
Gaetke, L., 2003. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology, [online] 189(1–2), pp.147–163. https://doi.org/10.1016/s0300-483x(03)00159-8.
Galaris, D., Barbouti, A. and Pantopoulos, K., 2019. Iron homeostasis and oxidative stress: An intimate relationship. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, [online] 1866(12), p.118535. https://doi.org/10.1016/j.bbamcr.2019.118535.
Greenberg, G.R. and Wintrobe, M.M., 1946. A LABILE IRON POOL. Journal of Biological Chemistry, [online] 165(1), pp.397–398. https://doi.org/10.1016/s0021-9258(17)41250-6.
Gutteridge, J.M.C. and Halliwell, B., 2018. Mini-Review: Oxidative stress, redox stress or redox success? Biochemical and Biophysical Research Communications, [online] 502(2), pp.183–186.
https://doi.org/10.1016/j.bbrc.2018.05.045.
Harris, Z. L., Durley, A. P., Man, T. K., & Gitlin, J. D. (1999). Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proceedings of the National Academy of Sciences of the United States of America, 96(19), 10812-10817. https://doi.org/10.1073/pnas.96.19.10812
Hentze, M.W., Muckenthaler, M.U., Galy, B. and Camaschella, C., 2010. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell, [online] 142(1), pp.24–38. https://doi.org/10.1016/j.cell.2010.06.028.
Jeong, S.Y. and David, S., 2003. Glycosylphosphatidylinositol-anchored Ceruloplasmin Is Required for Iron Efflux from Cells in the Central Nervous System. Journal of Biological Chemistry, [online] 278(29), pp.27144–27148. https://doi.org/10.1074/jbc.m301988200.
Ke, Y. and Qian, Z.M., 2007. Brain iron metabolism: Neurobiology and neurochemistry. Progress in Neurobiology, [online] 83(3), pp.149–173. https://doi.org/10.1016/j.pneurobio.2007.07.009.
Kenkhuis, B., Bush, A.I. and Ayton, S., 2023. How iron can drive neurodegeneration. Trends in Neurosciences, [online] 46(5), pp.333–335.
https://doi.org/10.1016/j.tins.2023.02.003.
Kruszewski, M., 2003. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, [online] 531(1–2), pp.81–92.
https://doi.org/10.1016/j.mrfmmm.2003.08.004.
Milanino, R., Conforti, A., Franco, L., Marrella, M. and Velo, G., 1985. Review: Copper and inflammation — a possible rationale for the pharmalogical manipulation of inflammatory discorders. Agents and Actions, [online] 16(6), pp.504–513. https://doi.org/10.1007/bf01983655.
Mills, E., Dong, X., Wang, F. and Xu, H., 2009. Mechanisms of Brain Iron Transport: Insight into Neurodegeneration and CNS Disorders. Future Medicinal Chemistry, [online] 2(1), pp.51–64. https://doi.org/10.4155/fmc.09.140.
Moos T., Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 2000 Feb;20(1):77-95. doi: 10.1023/a:1006948027674. PMID: 10690503.
Moos, T., Nielsen, T.R., Skjørringe, T. and Morgan, E.H., 2007. Iron trafficking inside the brain. Journal of Neurochemistry, [online] 103(5), pp.1730–1740. https://doi.org/10.1111/j.1471-4159.2007.04976.x.
Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr. 2008;28:197-213. doi: 10.1146/annurev.nutr.28.061807.155521. PMID: 18489257.
Prohaska, J. R. (2011). Impact of Copper Limitation on Expression and Function of Multicopper Oxidases (Ferroxidases). Advances in Nutrition, 2(2), 89-95. https://doi.org/10.3945/an.110.000208
Sorenson, J.R.J., 1989. 6 Copper Complexes Offer a Physiological Approach to Treatment of Chronic Diseases. Progress in Medicinal Chemistry, [online] pp.437–568. https://doi.org/10.1016/s0079-6468(08)70246-7.
Uriu-Adams, J.Y. and Keen, C.L., 2005. Copper, oxidative stress, and human health. Molecular Aspects of Medicine, [online] 26(4–5), pp.268–298. https://doi.org/10.1016/j.mam.2005.07.015.
Vashchenko, G., & A. MacGillivray, R. T. (2013). Multi-Copper Oxidases and Human Iron Metabolism. Nutrients, 5(7), 2289-2313. https://doi.org/10.3390/nu5072289
Wang, J. and Pantopoulos, K., 2011. Regulation of cellular iron metabolism. Biochemical Journal, [online] 434(3), pp.365–381. https://doi.org/10.1042/bj20101825.
Wallander, M.L., Leibold, E.A. and Eisenstein, R.S., 2006. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, [online] 1763(7), pp.668–689. https://doi.org/10.1016/j.bbamcr.2006.05.004.
Ward, R.J., Zucca, F.A., Duyn, J.H., Crichton, R.R. and Zecca, L., 2014. The role of iron in brain ageing and neurodegenerative disorders. The Lancet Neurology, [online] 13(10), pp.1045–1060.
https://doi.org/10.1016/s1474-4422(14)70117-6.
Wang,J Kostas Pantopoulos; Regulation of cellular iron metabolism. Biochem J 15 March 2011; 434 (3): 365–381. doi: https://doi.org/10.1042/BJ20101825
Allergy-Friendly Pets
Key Highlights Hypoallergenic pets are great for people with pet allergies, as they produce fewer allergens like dander, saliva, and proteins that can trigger symptoms….
Chronic Kidney Disease (CKD): Causes & Treatment
Key Takeaways Ultra-processed foods and high carbohydrate intake worsen inflammation, harming kidney function. Iron overload leads to oxidative stress, which accelerates CKD progression. Copper is…
SIBO Bloating: Causes, Diet, & Management Tips
Key Takeaways SIBO disrupts gut bacteria balance, causing bloating, pain, and nutrient absorption issues. Symptoms include bloating, abdominal pain, diarrhea, constipation, weight loss, and fatigue….
7 Remedies for Kidney Stones: A Comprehensive Guide
Key Takeaways Staying well-hydrated and adopting a balanced diet can help prevent kidney stones. Knowing the causes of kidney stones can inform effective prevention strategies….
Alzheimer’s Disease: Symptoms, Causes, Treatment
Key Takeaways Alzheimer’s disease is a progressive neurodegenerative disorder affecting memory, thinking, and behavior. Oxidative stress, including from excess iron, plays a significant role in…
How to Lower Triglycerides Fast: Natural Solutions
Key Highlights Triglycerides, a type of fat found in the blood, are essential indicators of metabolic health. Elevated triglyceride levels increase the risk of heart…
Metabolic Health: What It Means and How to Improve It
Key Takeaways Metabolic health reflects how well your body processes energy and maintains stable blood sugar, cholesterol, and blood pressure. Key indicators of metabolic health…
Remnant Cholesterol (RC): Its Origins & Impact
Key Takeaways Remnant cholesterol (RC) is the cholesterol content left in the blood after triglycerides are removed from VLDL and IDL particles. RC is a…
Atherosclerosis Prevention Strategies: Insights from Scientific Research
Key Takeaways Atherosclerosis is the hardening and narrowing of arteries caused by plaque buildup. Chronic inflammation and oxidative stress contribute to the development of plaque….
Postbiotics: What They Are and Why They Are Important
Key Takeaways Postbiotics 101: They’re beneficial by-products from probiotics that consume prebiotics Boosts Immunity: Postbiotics sharpen your immune system, helping fight off pathogens and reducing…
Coping with Pet Allergies: Tips & Advice
Key Highlights Pet allergies often cause sneezing, coughing, itchy eyes, and skin rash. Pet allergens are in the saliva, urine, and dander of furry animals….
Non-Alcoholic Fatty Liver Disease (NAFLD)
Key Takeaways NAFLD involves fat buildup in the liver not caused by alcohol. Commonly associated with obesity, insulin resistance, and metabolic syndrome. NAFLD can lead…
Diabetes: Everything You Need to Know
Key Takeaways Type 1 and Type 2 diabetes involve insulin regulation issues, with Type 2 being the most common due to insulin resistance. Copper, retinol,…
Proteolytic Enzymes and Heart Health: What the Research Shows
Your heart works tirelessly to pump blood throughout your body, delivering essential nutrients and oxygen to your cells. However, factors like poor diet, stress, and…
Parkinson’s Disease : Symptoms, Causes & Treatment
Key Takeaways Parkinson’s disease is a progressive neurological disorder that affects movement and coordination. Oxidative stress and excess iron are significant factors in the progression…
Supporting Mental Health with Gut Health
Key Takeaways Gut-Brain Connection: Gut health is directly linked to mental wellbeing through the gut-brain axis. Probiotics: Beneficial bacteria that help regulate mood and support…
Uric Acid: Effects & Management
L-Glutamine and Gut Health: Benefits and Side Effects
Key Takeaways L-Glutamine is essential for gut health. Benefits include improved digestion and reduced inflammation. Potential side effects are rare but can occur in high…
Vegetable Oil: Health Risks You Might Not Know
Key Takeaways: Omega-6 fats from vegetable oils cause oxidative stress and inflammation. Reducing omega-6 intake and using stable fats can lower health risks. High triglycerides…
Travel Hygiene Tips: Stay Fresh on the Go
Key Highlights Key practices include frequent handwashing, showering, and oral care. Packing a portable hygiene kit can help you stay fresh on the go. Advanced…
Quit Sugar for 14 Days: What Happens to Your Body?
Key Takeaways: Immediate Health Benefits of Reducing Sugar: In just two weeks, enjoy enhanced energy levels, weight loss, a reduced risk of chronic diseases, and…
7 Simple Tips for Lowering Blood Pressure Naturally
Maintaining healthy blood pressure levels is essential for overall well-being, as high blood pressure can lead to serious health complications. However, it is possible to…
Dialysis: Benefits & Challenges
Key Takeaways Dialysis removes waste and excess fluid from the blood when kidneys cannot function. Two main types: hemodialysis (machine-based) and peritoneal dialysis (abdomen-based). Dialysis…
Inflammation: Causes & Effects
Key Takeaways Inflammation is the body’s response to injury or infection, but chronic inflammation can lead to health problems. Iron overload from artificial sources and…
Alcohol and Its Effects
Key Takeaways Alcohol is metabolized primarily in the liver, producing acetaldehyde, a toxic byproduct. Chronic alcohol consumption leads to liver damage, including fatty liver, hepatitis,…
Histamine: What You Should Know
Key Takeaways Histamine’s Role: Vital in immune responses, digestion, and as a neurotransmitter in the central nervous system. Histamine Production: Produced in mast cells and…
Is Eating Sugar Really That Bad For Your Health?
Should You Really Be Concerned? In short, YES! Thank you, that’s all folks, and do have a good evening. Seriously though, extensive research has established…
Triglycerides: Levels & Range Explained
Key Highlights Triglycerides are the most common form of fat in the body play a role in energy storage High levels of triglycerides can increase…
Insulin Resistance: What It Is & How to Manage It
Key Takeaways Insulin resistance leads to high blood sugar when cells stop responding to insulin. Often connected to obesity, poor diet, and physical inactivity. Symptoms…
Metabolic Syndrome: Managing This Health Risk
Key Takeaways Metabolic syndrome is a cluster of conditions increasing the risk of heart disease, stroke, and diabetes. Symptoms include high blood pressure, high blood…
Boost Insulin Sensitivity Naturally
Key Takeaways Improving insulin sensitivity helps control blood sugar and reduces the risk of metabolic disorders. Regular physical activity enhances how cells respond to insulin….
High Homocysteine: How to Manage Levels
Key Takeaways: Elevated homocysteine can raise the risk of heart disease and other health problems. Animal-based foods high in B vitamins help reduce homocysteine levels….
Signs of Diabetes: Recognizing the Red Flags
Key Takeaways Increased Thirst and Urination: High blood sugar leads to dehydration, causing excessive thirst and frequent urination. Unexplained Weight Loss: Diabetes can cause the…
High Homocysteine: How to Manage Levels
Key Takeaways: Elevated homocysteine can raise the risk of heart disease and other health problems….
Real Food for Pregnancy by Lily Nichols
Key Takeaways Evidence-Based Guidance: Challenges outdated prenatal nutrition with researched alternatives. Nutrient-Dense Foods: Stresses eating…
Change Your Diet, Change Your Mind by Dr. Georgia Ede
In the compelling book Change Your Diet, Change Your Mind, Dr. Georgia Ede challenges conventional…
Increase GLP-1 Agonists Naturally
Key Takeaways: GLP-1 agonists regulate appetite, insulin production, and blood sugar levels. Regular exercise and…
Osteoarthritis Symptoms & Home Remedies
Key Takeaways Lifestyle adjustments and alternative therapies contribute to overall symptom management. Low-impact exercises and…
Potassium: Benefits & Sources
Key Takeaways Potassium is essential for regulating fluid balance, nerve signals, and muscle function. It…
GABA (gamma-aminobutyric acid)
Key Takeaways GABA is a neurotransmitter that helps calm the nervous system. Low GABA levels…
TUDCA Benefits for Health
Key Takeaways TUDCA promotes liver health, aiding cell protection and repair. Enhances digestion by improving…
Metabolic Health: What It Means and How to Improve It
Key Takeaways Metabolic health reflects how well your body processes energy and maintains stable blood…
The Plant Paradox by Dr. Steven Gundry
Key Takeaways Lectins as Toxins: Lectins can cause inflammation and various health issues, Dr. Gundry…
Sustainable Beauty: Redefining Skincare with Eco-Friendly Practices
Key Highlights Sustainable beauty focuses on products that are good for the planet and consumer…